
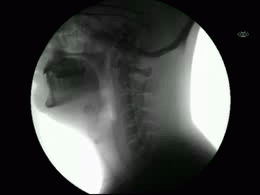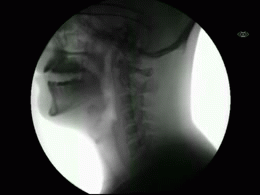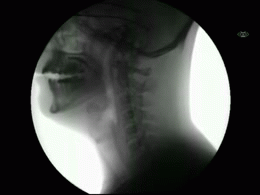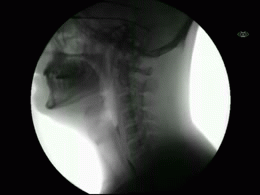
This newly released study represents the most extensive collection of swallowing evaluations in NPC1 to date. This large data series can serve as a foundation for NPC1 swallowing and speech domains assisting clinical management, quality of life decisions, and therapeutic outcomes for future clinical trials. Utilizing videofluoroscopic swallowing study (VFSS), also known as the modified barium swallow study, to visualize swallowing function directly, the study results identify NPC1 swallowing impairments, including silent aspiration often missed in clinical swallowing examinations. Thus, an interpretive quantitative analysis of VFSS was applied to NPC1 swallowing functioning and aspiration risk, facilitating clinical management. The study results describe a non-linear, heterogeneous rate of swallowing decline with the primary outcomes in its patients. Additionally, the variables of seizure history and the length of time an individual participated in the NIH study, were observed to significantly increase the risk of swallowing decline, while the use of the drug Miglustat demonstrated a protective effect—previously identified in published studies. This study revealed Miglustat’s protection of swallowing function and its positive effects on speech and oral-motor functions, encouraging its clinical use among NPC1 patients.
The researcher’s analysis and statistical modeling from the NIH natural history study provide an improved understanding of NPC1 disease progression with swallowing function. Additionally, the researchers concluded that these swallowing assessments and analysis should be considered for other rare disease research.
Authors: Beth I. Solomon, Andrea M. Muñoz, Ninet Sinaii, Nicole M. Farhat, Andrew C. Smith, Simona Bianconi, An Dang Do, Michael C. Backman, Leonza Machielse & Forbes D. Porter
Published September 5, 2022

